LEGFLOW RX/OTW®
Paclitaxel Releasing Peripheral
Balloon Dilatation Catheter
The revolutionary efficacy in SFA and BTK revascularization!
LEGFLOW RX/OTW provides a 100% anti-proliferative vessel wall protection,
and promotes rapid healing after mechanical balloon angioplasty.
The best option for SFA/popliteal de novo and restenotic lesions.
Treating peripheral artery disease (PAD) in the superficial femoral artery (SFA) is a difficult challenge. The anatomy is very dynamic and contains complex lesions, but also patient comorbidities such as diabetes require the clinically most-effective therapy. The new LEGFLOW Paclitaxel-releasing peripheral balloon dilatation catheter provides a clinically most successful treatment of peripheral artery disease, and ensures a long-term vessel patency.
Please learn more about our ongoing trials and growing body of evidence.
The new treatment of BTK lesions and critical limb ischemia! LEGFLOW shows promising early clinical study results.
The rate of recurrent stenosis after PTA and stenting is higher in the below-the-knee area than in femoropopliteal procedures. The absence of metal struts makes the LEGFLOW balloon dilatation suitable for treating long lesions, especially in small diameter vessels and areas in which flexion and compression of the stent may occur and result in stent fractures, particularly in the below-the-knee area. Thanks to the new stable ‘SAFEPAX’ PTX coating technology, the treatment of critical limb ischemia patients appears as safe as POBA angioplasty.
Clinical cases
Prohealing peripheral interventions with much less
restenosis. Successful treatment with anti-proliferative
vessel wall protection.
- Conventional Mechanical Balloon Angioplasty
- Trauma
- Restenosis
- Complications
- Short- term Repeat Intervention
- High Patient Cost
- Angioplasty with Prohealing Local Drug Delivery
- Trauma
- Inhibition of Proliferation
- Healing
- Less Repeat Intervention
- Health Care Saving

Safe Drug Coating Technology for a clinically advanced patient treatment quality.
High coating stability and
minmal particulate loss.
Perform precedures with no need to rush.
The lowest PTX serum concentration on the market.
LEGFLOW ‘SAFEPAX’ coating is a step beyond contemporary first and second generation DCB coatings which had to compromise on vulnerable balloon coating mixtures out of a high water soluble drug excipient with relatively large PTX crystals.

Microscopy of the LEGFLOW DCB balloon surface
showing no visible PTX particulates
Minimizing the risk of microembolization.
The LEGFLOW ‘SAFEPAX’ drug coating technology provides a maximum protection from downstream micro-embolization effects to minimize the risk potential of any thrombotic event. The biological ’SAFEPAX’ drug release matrix avoids the side effects of plasticizer or contrast media drug excipients. Especially for the treatment of lower limb ischemia patients, the ‘SAFEPAX’ PTX drug coating of Legflow minimizes any undesirable risk of pro-thrombotic side effects, which might be associated with Paclitaxel.

‘Stable’ vs. ‘unstable’ DEB PTX coating
When invisibility equals safety!
1. LEGFLOW DCB PTX non crystalline coating.
2. Other DCBs with ‘unstable’ and brittle balloon coatings, consisting of large 2.0µm-3.0µm (visible) PTX crystals, bear a risk for the physician and patients.
About Safepax
Proven! Optimal therapeutic drug-in-tissue bioavailability resulting
in maximal clinical efficacy.
No signs of vessel toxicity were found. No other safety concerns were noted in animals studied for up to 90 days.*
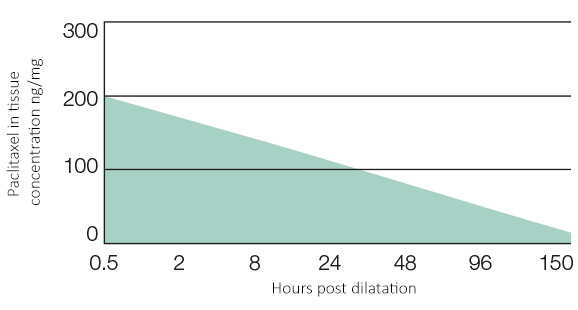
A short-term balloon-to-vessel wall contact time of 90-120 sec. is sufficient to inhibit SMC proliferation sustainably for up to 150 hours.
Pre-clinical results confirm no noticeable embolization results similar to POBA. Pre-clinical study results confirm a most effective drug delivery into the vascular tissue, showing a sustained drug effect of up to 28 days. ‘Cardionovum’s DCB shows great effectiveness in neo-intimal growth reduction in diseased vessels.’
*Source: Report on pre-clinical studies performed
at CV-PATH Institute (R. Virmani MD), Gaithersburg USA
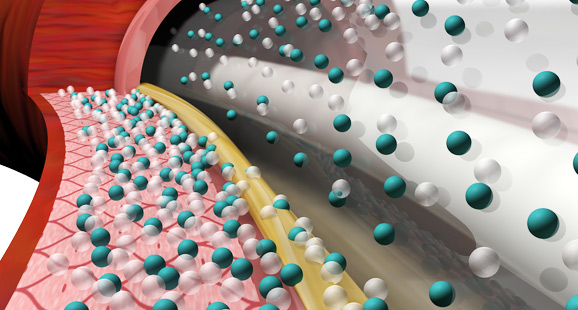
LEGFLOW SAFEPAX performance. Effective DCB PTX drug-transfer.
Consistent and predictable drug delivery to the artery lesion site results in a homogenous and maximized drug absorption into the arterial tissue.
LEGFLOW ensures a high patient safety by non-crystalline coating ahead of 2.0-3.0µm large brittle Paclitaxel crystals on most other DCBs.
>80.000 LEGFLOW used so far
4 RCTs with +288 pts enrolled
fatal event related to Legflow
Available in three platforms, OTW and RX, design, over 70 sizes and configurations. The best option for a successful treatment of any SFA and BTK indication.

Treatment of SFA:
4.0 mm - 10.0 mm balloon diameter
1350 mm catheter length
Treatment of BTK:
2.0 mm - 4.0 mm balloon diameter
1500 mm catheter length




