XLIMUS®
Sirolimus Eluting
Coronary Stent System
Dr. Luca Testa from Italy presents clinical outcomes of the XLIMIT Trial.
A randomized controlled trial to assess endothelialization.
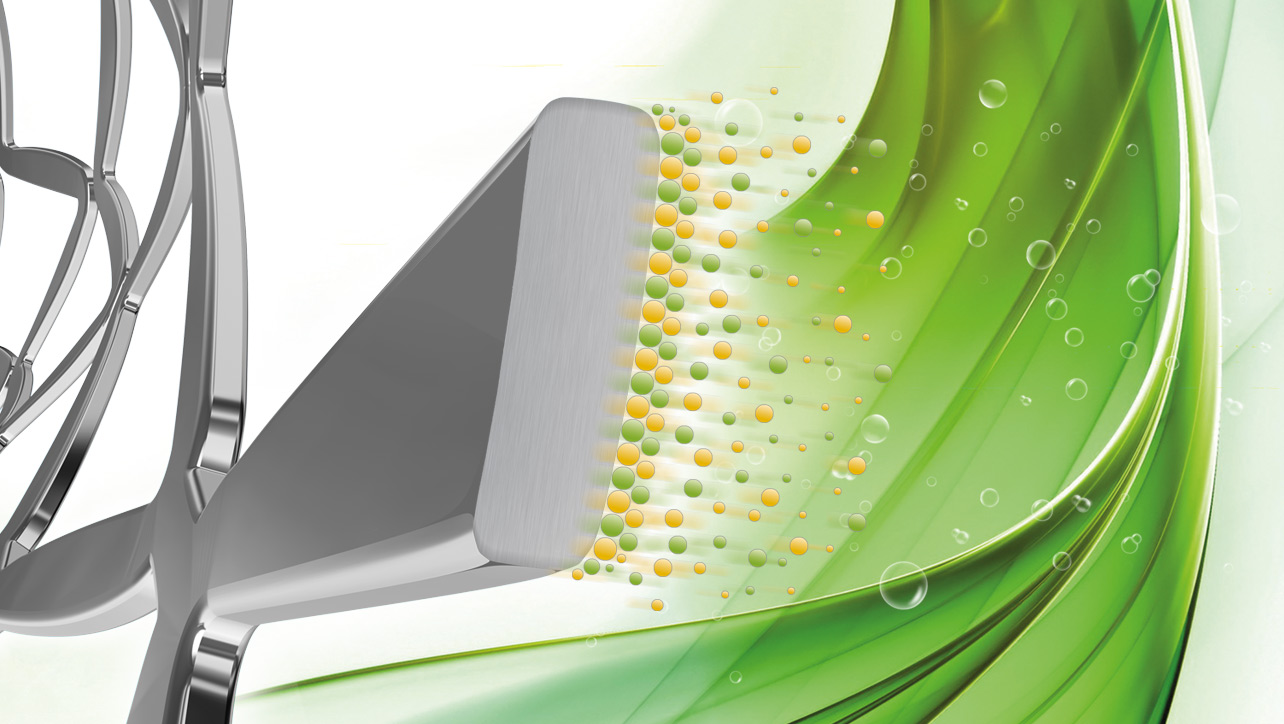
XLIMUS DES provides fully biodegradable drug elution.
No stent flaring. No tissue prolapse. Clinically effective.
The unique XLIMUS 6-8-10 stent cell design.
Ensures even vessel wall coverage. Any different artery lesion diameter ranging from 2.25 up to 5.00mm is stented evenly.
Homogenous, clinically effective drug delivery optimizes the anti-proliferative protection of the stented lesion segment.
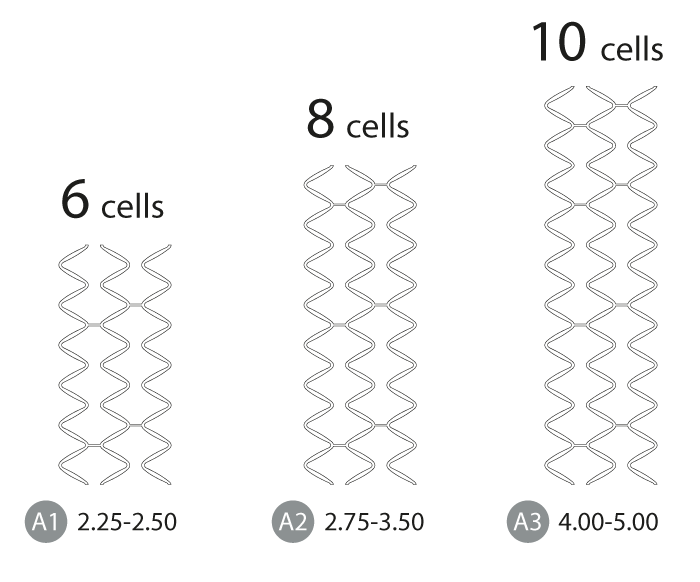
Homogenous deployment distribution: Surface A1 = A2 = A3
No stent strut flaring. No open gaps.
The technically high standard of 6-8-10 intermediate and closed-cell stent architecture covers all vessel diameters evenly. XLIMUS quality ensures the best possible intracoronary stenting stability and minimizes stenting trauma and restenosis. Extraordinary homogenous vessel wall scaffolding.
XLIMUS assists the cardiologist
with an optimal, unsurpassed tracking performance. It has an innovative Hydrophilic-coated shaft and an extra-low tip profile to access the most tortuous lesions. The ultra-low lesion crossing profile measures only 0.90 mm. The novel XLIMUS Sirolimus-eluting coronary stent system protects the stented lesion segment through extraordinary homogeneous vessel wall scaffolding which minimizes the risk of tissue prolapse and optimizes Sirolimus drug distribution.
Following the nature. Stent flexibility by design.
Controlled biodegradable Sirolimus drug release for
rapid functional endothelial healing.
No signs of vessel toxicity were found. No other safety concerns were noted in animals studied for up to 90 days.

Reliable fully biodegradable Sirolimus drug release
for rapid functional endothelial healing.
The highly biocompatible PLLA (Polylactid acid) drug containing release matrix degrades smoothly and provides an optimal release kinetic profile. Within 30 days, about 70% of the anti-proliferative drug is distributed into the surrounding arterial tissue of the stent struts, ensuring a highly effective inhibition of smooth muscle cell migration and proliferation. Pharmacokinetic study result confirm sustained anti-proliferative drug efficacy up to 120 days.
| Cypher | 140 µm |
| Taxus Liberte | 97 µm |
| Endeavor | 91 µm |
| Xience V | 81 µm |
| XLIMUS | 71 µm |
Thin stent struts minimize foreign body metal volume.
Pulse Synchronous Stent Dynamics respond to coronary artery movement, with every heart beat. Natural stent flexion minimizes friction and shear stress to avoid vessel wall trauma. For a lifetime patient safety! XLIMUS reduces the inflammatory signal potential for prevention of late restenosis.
Source: Peter Smits,MD, from the COMPARE trial presentation at TCT 2009.